Introduction
Finding
solutions to problems requires identifying their causes. Without that, only
symptoms are likely to be addressed, leaving the root causes to generate
further problems. The lack-of-reproducibility crisis in biomedical research is
one of the indications that modern medicine in the most advanced countries has
gone wrong in recent decades, as described and documented in many books and
articles (1). In addition to publication of those works, reactions to what has been
going wrong include an initiative toward explicitly evidence-based medicine
(2-4) and founding of the Journal of Controversies in Biomedical Research.
Medicine
has gone wrong through the synergy over many decades of several developments,
some intellectual and some commercial or practical. Most fundamental is the
failure to distinguish, in principle and in treatment, between infectious and
non-infectious, innate, constitutional conditions. Diseases caused from outside
the affected individual are different in kind from undesired health conditions
arising from internal, inherent physiological processes, and they should be
addressed differently. That fundamental failure to distinguish between
basically different types of conditions has been exacerbated by the
well-intentioned urge to make medical practice more scientific (illustrating
the old saw that the road to hell is paved with good intentions). It had seemed
reasonable to seek objective and quantitative measures of ill health as guides
to diagnosis as well as treatment, so a large range of tests has become
routine: blood pressure (BP), blood sugar, cholesterol levels, PSA, etc., etc.
Those
measures are symptoms, but they came to be identified as the causes of ailments
or even as those conditions themselves. Labeling away-from-average levels of
BP, blood sugar, cholesterol, etc., as “risk factors” is readily misinterpreted
to mean that modifying them could modify actual risk. That postulates a
causative relationship between the measured quantity and the undesired
condition of which it is a symptom, when all that is known is that there exists
a statistical correlation — and correlation never proves causation. That
confusion of correlation with causation, and of symptom with disease, became
even more explicit as these measures came to be also called “surrogate markers”
or “biomarkers” of disease. First, high BP and high levels of cholesterol were
taken on the basis of statistical correlations to be biomarkers of
cardiovascular disease (CVD) as well as risk factors for CVD. That led to
administering drugs to lower BP as though high BP were itself CVD or actually
caused CVD. The misinterpretation did not become quickly evident because the
success of treatment was judged by the effect on BP, not on the actually desired
outcome which is a decreased morbidity and mortality from CVD, lesser incidence
of heart attacks and strokes.
Things
have also gone wrong in how clinical trials are carried out, and especially in
how results are analyzed and interpreted. There are innumerable pitfalls in the
designing and carrying out of clinical trials — biased sampling, inappropriate
control groups, many more. But there is also an overarching misapplication of
the statistical analyses that guide actual medical practice: namely, the
criterion for “statistical significance” is quite weak, and it does not reflect
how large a supposed benefit might be; nor is that hoped-for benefit
systematically compared to the risks of “side” effects. The deficiencies in the
statistics are demonstrable not only on first principles, their consequences
have shown up in practice in the fact that, increasingly in recent decades,
approved drugs have had to be withdrawn from the market (5) at shorter and
shorter intervals (6) after the initial approval.
The
presumption that non-infectious conditions can be properly treated in the same
manner as infectious ones (by “magic bullet” drugs), the confusion of
correlation with causation, and the flaws in statistical interpretation have
led to a huge increase in consumption of prescription drugs, bringing an
enormous expansion of the pharmaceutical industry (“Big Pharma”), which has
become the most profitable of all industries (7-9). That has led to pervasive
conflicts of interest which have corrupted research and emasculated regulation.
Addressing the overall problem or any of its major symptoms will require
efforts to deal with all of these causes. Unfortunately they are not entirely
independent of one another, partly as a result of pervasive conflicts of interest,
partly because Big Pharma will actively oppose anything that threatens to
restrict even inappropriate or illegal prescribing of drugs.
Infectious and
non-infectious conditions
When
illness is caused by something invading a host, one may reasonably hope that it
is possible to kill the invader without harming the host; perhaps one could
find a substance (a “magic bullet”) that exploits differences between the
physiologies of invader and of host. That is not a reasonable hope with viruses
since their chemistry is so much like that of animal cells, and the most
effective guard against viruses is by vaccination. Against bacteria, various
chemicals and particularly antibiotics have been very successful. Although none
is fully lethal to the invader while completely harmless to the host, undesired
“side” effects can be managed by keeping the duration of treatment very short,
typically on the order of days or weeks. By contrast, chronic conditions not
caused by invading entities require lifelong treatment.
Inflammation,
cancer, cardiovascular disease, and organ dysfunctions are not caused by
identifiable invading entities. They arise because something has gone wrong in
the body’s physiology. That physiology is an excruciatingly complicated
interconnected system of signals and feedbacks and reactions which under normal
circumstances maintains an extraordinarily stable set of conditions. There is
no a priori reason to imagine that
normal stability could be regained after a system dysfunction by administering
a single substance or even a few. Yet present-day treatment of chronic,
non-infectious ailments is based on this unlikely premise. That approach is not
only without a reasonable basis in theory, it is also hazardous in practice
because the treatment of chronic ailments is not restricted to short periods as
with infectious diseases, it is intended to be lifelong. “Side” effects may
cumulate very harmfully when drugs are taken without interruption for long
periods, as with BP-lowering medications or blood-sugar controllers or
cholesterol-lowering drugs.
The
scare quotes on “side” effects are intended to emphasize that chemicals do not
know what we want them to do, they just exert their chemical effects. Current
advertisements for prescription drugs typically list a whole host of really
dangerous “side” effects, albeit either in very fine print in pamphlets or on
television in cheery voices as the screen shows joyful doings of happily
smiling people, overwhelming any effect of those grave warnings of “side” effects.
TV ads for Symbicort for asthma, for example, illustrate its possible benefits
through grandparents interacting happily and actively with grandchildren. The
truth is that it promises only that it “may lead to better breathing” (NB
“may”), while possible serious “side” effects include increased risk of
hospitalization or death from asthma (the condition supposed to be treated),
pneumonia, serious allergic reactions, decreased immune function, adrenal
insufficiency, more wheezing, glaucoma, cataracts, lower bone density, swelling
of blood vessels; as well as some common and “non-serious” “side” effects:
“nose and throat irritation, headache, upper respiratory tract infection, sore
throat, sinusitis, stomach discomfort, flu, back pain, nasal congestion, vomiting,
and thrush in the mouth and throat” (10).
Making medical practice
objective
Much has been learned by measuring a host of physiological variables. Nowadays blood tests report the levels of a great variety of substances, and measuring BP is routine at every visit to a doctor (despite the well-known fact that BP rises owing to stress associated with having to consult a doctor). The devil is in the detailed ways in which BP and other measures are interpreted, and the treatments to which they lead more or less routinely. How things started to go wrong has been detailed by Greene (11). BP had been measured since early in the 20th century. Insurance companies learned of and archived these measurements since physical examinations were required when applying for life insurance. The cumulated data showed that high BP correlated with earlier death. Now that is a perfectly reasonable actuarial basis for adjusting life-insurance premiums, whether or not high BP is actually a cause of earlier death. It is not reasonable, though, to extrapolate from that to the idea that lowering BP for an individual will increase that person’s lifespan. Yet that extrapolation has guided medical practice for half a century. The early measurements of BP had also revealed that it rises with age. A rule of thumb half a century ago was that normal systolic BP equals age plus 100. Much data accumulated over the decades indicate that this is not far from the truth; perhaps a slight over-estimate, it may be more like 100 plus 80-90% of age (12) (Figure 1).
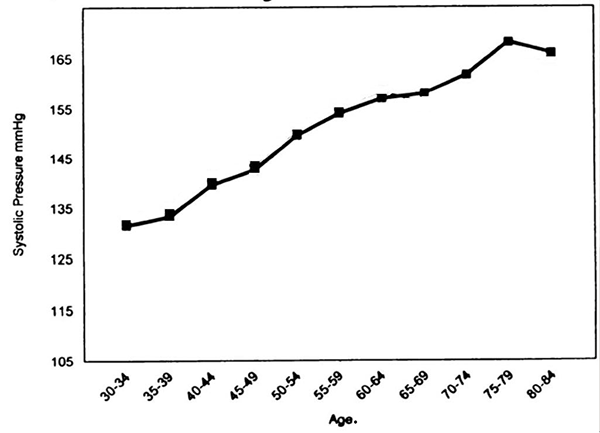
A
very similar trend is shown in data at MedIndia (13). From the late 20s to the
late 70s, normal systolic BP rises at something like 1 mmHg for each year of
life. But the exact magnitude of the rise with age is irrelevant. The important
point is just that BP increases normally with age. The risk of death also
increases normally with age. Thus the risk of death must correlate with BP
simply because both of them correlate with age. That does not suggest that
lowering BP at any age is necessarily beneficial. Yet current medical practice
ignores the natural increase of BP with age, and arbitrarily sets the limits of
high BP or
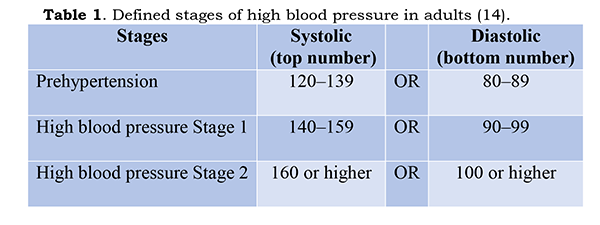
The
failure to recognize that BP rises normally with age becomes beyond absurd with
the statement that “prehypertension can progress to high blood pressure”. As
presently defined, it will do so quite normally over the years. Another very
serious fallacy in defining unhealthy BP in this manner is that these numbers
assert that every individual should be at the population average. The same
fallacy pertains to interpretation of all the other factors commonly tested:
cholesterol, blood sugar, etc. In point of fact, most physiological factors
vary appreciably within healthy populations. For BP, the standard deviation for
apparently healthy people suggests that variations of BP by +/- 15% or so are
within the normal range (15) and should not be regarded as abnormal or
unhealthy or cause for concern. Under present guidelines, a large proportion of
upper-middle-aged and older people are defined as having hypertension; 75-80%
of Americans aged above 60, according to the Institute of Medicine (16).
Present practice is to prescribe for such “hypertension” supposedly preventive
BP-lowering medications, all of them with undesirable “side” effects. This
practice is misguided (17, 18). Greene (11) continues from BP with the story of
how measuring numbers also led to drug-based treatments for “high” blood sugar
and “high” cholesterol. The same lack of allowing for individual variability
pertains here, as well as the confusion of correlation with causation.
Overall,
diagnosis and treatment have become increasingly guided by numbers from tests,
largely replacing individual clinical judgment based on patient’s feelings and
symptoms as observed and interpreted by physicians. Doctors are increasingly in
groups, clinics, and large organizations, and that makes it even more difficult
for individual physicians to use individual judgment, since their actions are
being observed and sometimes even mandated to conform to accepted norms,
namely, standard average numbers and treatments.
Biomarkers, surrogate
markers
So
BP, blood sugar, cholesterol and many other measures became “biomarkers” or
“surrogate markers” of undesired conditions: heart disease and diabetes in
these cases, later such things as bone density as a measure of the risk of bone
fracture. These measures have been assumed to reflect accurately the status of
the diseases themselves. The evidence is, however, that treating biomarkers has
not brought the desired benefits. The surrogate markers are at best imperfect
correlates of their parent disease (16, 19, 20) and they are certainly not
causes (21): “There are no valid data on the “effectiveness” of “statins [in preventing heart attacks],
antihypertensives [in preventing heart attacks or strokes], and bisphosphanates
[to treat osteoporosis]”: because lowering cholesterol, lowering blood
pressure, and increasing bone density — the surrogate markers — have never been
shown respectively to result in fewer heart attacks or strokes or fewer bone
fractures.
Clinical trials
The
lack of reproducibility of results in medical science shows that what are
reported as results of clinical trials, and the conclusions drawn from them,
are not reliable. Part of the reason lies in how statistical analysis has been
applied in clinical trials, discussed in a later section. But there are a whole
host of other pitfalls in designing and carrying out clinical trials, and many
volumes have given chapter and verse about how often these have not been
avoided; in fact, protocols have too often been deliberately designed to favor
a desired outcome. The three major points made and documented in these sources
(22-25) and elsewhere (1, 26-28) are listed below.
First,
trials enroll subjects not representative of those for whom a drug will later
be prescribed. For example, very ill people are enrolled because beneficial
effects of a drug will show up more readily. But the drugs will later be
prescribed for others not nearly so ill and who may therefore not benefit. That
lowering BP for someone aged 35 with readings of say 220/100 may be beneficial
does not show that doing so for everyone over 140/90 is beneficial. Second,
safety of a drug is first tested on people who are not ill. Individuals are
enrolled by offering incentives that may be very attractive to poor, homeless, or
unemployed people. Enrolling in clinical trials has actually become regular
employment for some people. Those who arrange these tests come to know which
individuals are unusually healthy and least affected by drug “side”-effects,
and they are enrolled preferentially. So the incidence of “side” effects as
reported for drug approval may be considerably lower than when the drug is
taken by the general public, let alone by patients who are already ill. Third, drugs are not tested by independent
investigators. Now-a-days in developed countries it is done typically by
commercial Contract Research Organizations (CROs), whereas it used to be done
by academic and clinical institutions with funding from drug companies. In both
circumstances, the drug companies inevitably exert influence to make the
results favorable to them. With academic and clinical institutions, the
contracts typically contain language making the results confidential and giving
the drug company control over the publication of results. The clients of CROs
are the drug companies, neither the general public of prospective patients nor
the members of the practicing medical profession, and a CRO gets future benefit
from delivering results that please its clients.
There
are many ways to bias trial protocols to get a favorable rather than an
unfavorable result, and all of these are known to have been used at times: bias
the choice of trial subjects and control groups; do a preliminary test and then
drop from the later “actual” trial any subjects who have not benefited or who
have developed bad side-effects; stop a trial at a point where the results look
good even if the protocol called for a longer trial; and test a new drug
against something that will ensure a favorable result (29). For instance, test
a new drug against unusually high or low doses of a competing comparison drug —
high doses to increase “side” effects, low doses to decrease efficacy.
Approving new drugs
The
flaws in clinical trials are exacerbated by regulations that are far too lax to
ensure that drugs are both safe and effective. The Food and Drug Administration
(FDA) requires only two successful (statistically significant) trials lasting
at least 6 months. The FDA does not require results of all trials to be
submitted. The results submitted for approval may have been selected from a
larger number of unsuccessful trials. Six months is far too short a time to
assure the safety of drugs intended to be taken lifelong, namely to treat BP,
blood sugar, cholesterol, etc. The criterion for effectiveness of a drug is
based on biomarkers. That does not demonstrate benefits against the actual
disease. Trials are not aimed at finding the lowest useful dose of a drug,
because drug companies have no interest in that; whereas patients would benefit
from using the lowest beneficial dose because that decreases the chance of
harmful “side” effects. Once a drug has been approved, there is no systematic
monitoring of how it performs in practice. If seriously damaging “side” effects
turn up, that may not come to official attention and lead to official action
until an appreciable number of people have been clearly harmed, perhaps to the
point of death.
The
inadequacy of the present way of approving drugs is illustrated by the fact
that newly approved drugs have had to be withdrawn from the market (30) after
shorter and shorter times in the last few decades (6). As one result of the
lack of systematic official monitoring, adverse effects often become known to
consumers and lawyers before regulatory actions are taken. Drugs continue to be
advertised by the manufacturers at the same time as law firms are canvassing
for people to join class-action suits based on harm from the drugs’ “side”
effects, for instance on television in the USA in June 2015 with the anticoagulants
Pradaxa and Xarelto; in September 2015 with Invokana for type 2 diabetes. That
new drugs are approved when they are far from safe is also illustrated by the
fact that prescription drugs are the 3rd or 4th leading
cause of death in advanced countries (24, 25).
Statistical analysis
The
almost universally used mode of statistical analysis in medical matters (and in
the social sciences) assumes that the variable of interest is distributed
“normally” (follows the error curve or normal or Gaussian distribution) and
takes a result as “statistically significant” if p ≤ 0.05. That means less than
a 5% chance, 1 in 20 that the result is not meaningful, that it came about
purely by chance. In other words, treatments including drugs are being called
safe and effective when that this is not the case for at least every 20th
drug; “at least” because a more rigorous technical analysis of the protocols
for clinical trials suggests that the p ≤ 0.05 criterion can lead to
conclusions that are wrong as much as 30% of the time (31).
But
even 1 in 20 would be far too weak a criterion to satisfy the traditional form
of the Hippocratic Oath, “First: do no harm”. The p ≤ 0.05 criterion does not
mean that there is only a 5% chance that an approved drug is not beneficial, it
means that at least 1 in 20 of approved drugs, or as many as 3 in 10 (31),
should never have reached the market in the first place, because they have no
demonstrated benefit and, like all drugs, have potentially harmful “side”
effects. Furthermore, that something is “statistically significant” says
nothing about how large the effect is, and that matters crucially. For example,
a trial of decent size (~20,000 patients) (32) showed that clopidogrel (trade
name Plavix) did better at preventing strokes and heart attacks than aspirin,
at p = 0.043, well within p ≤ 0.05. But the difference in efficacy was very
small: The incidence of adverse events with clopidogrel was 5.32%/year, for
aspirin 5.83%/year, a difference so small that it might not outweigh the greater
risk of adverse “side” effects from clopidogrel. When a trial enrolls a large
number of subjects, a statistically significant result can follow even from a
tiny or negligible difference in efficacy.
Using
statistical significance alone as a criterion for approving a drug makes no
sense. The most informative manner of reporting the performance of drugs for
the benefit of doctors and patients would be in terms of NNT (the number of
patients who have to be treated to show clear benefit to one patient) and NNH
(the number of patients who have to be treated to show clear harm to one
patient). For example, to prevent one heart attack through aspirin therapy over
a 2-year period, among people with no known heart disease, 2000 patients need
to be treated (NNT = 2000). But aspirin can also cause bleeding, NNH = 3333. So
the chance of benefit — very small to start with — is only about twice the
chance of harm (33). With statins for people with no symptoms of heart disease,
the potential harm clearly outweighs the possible benefits (Table 2). On the other hand, when
people already have heart disease, the benefit/risk ratio becomes less
unfavorable (Table 3).
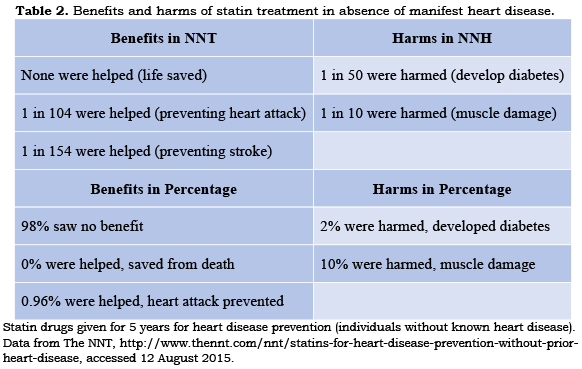
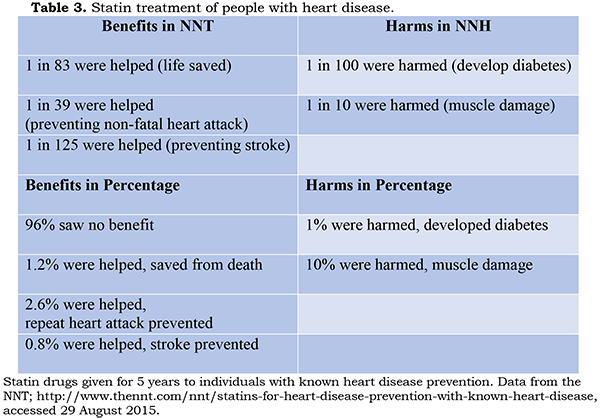
Still,
not every patient might choose to accept an 11% chance of harm in return for a
4% chance of protection from even a serious outcome; one’s family circumstances
and age would be a consideration. Moreover, a Mediterranean diet by contrast to
statins has been shown to lower the incidence of cardiovascular events (34).
But the point here is not to advise concerning statins. It is to demonstrate
that this way of presenting the data allows patients and doctors to arrive at
properly informed decisions. Under present circumstances, doctors and patients
are simply told by regulators and drug companies that a drug should be taken
because it has been pronounced safe and effective — after inadequate testing
and data analysis.
Because
this illuminating NNT/NNH way of describing benefit and risk of harm is not
commonly used, large numbers of people without heart disease are being
prescribed statins and suffering sometimes severe “side” effects without
compensating benefit; and similar conclusions probably apply to many other
currently standard treatments.
Big Pharma
Most
of the works listed in the cited bibliography (1) detail actions by drug
companies that are against the interests of patients and thereby against the
public good. Commonly mentioned are: illegally proselytizing off-label (not
approved) uses of drugs approved for a different purpose, despite fines on the
order of hundreds of millions, sometimes billions of dollars (35) (evidently
profits from the illegal marketing are significantly larger than the fines);
paying doctors and researchers to give “medical education” seminars that are
actually advertisements for a drug; paying doctors and researchers to put their
names on articles ghost-written by company staff; paying professional journals
to publish “Supplements” of articles favoring particular drugs. Merck had even
contracted with Elsevier to publish a spurious journal, Australasian Journal of
Bone and Joint Medicine, that had every appearance of a normal peer-reviewed
publication but that had simply been composed by Merck staff with articles
favoring their drugs (36); claiming the need for high drug prices to support
research when far more is spent on marketing than on research; and exerting
pressure on politicians through campaign contributions and lobbying, thereby
emasculating regulations and regulatory actions.
These
extraordinary charges, and more, are documented in considerable detail in a
number of the books listed in my bibliography (1). Peter Gotzsche, who directs
the Nordic Cochrane Center (37), even describes Big Pharma as “Organised Crime”
in the title of his book (24). Lest anyone doubt that commercial enterprises
could act so much against the common good, including in ways that verge on or
actually are criminal, Gotzsche (24) and Healy (25) both compare Big Pharma to
the tobacco industry, which has been shown publicly to put its profits ahead of
the health of its customers. Tobacco executives knew for decades of the health
dangers of smoking and that it is addictive, long before there came public
campaigns against smoking. In testifying to Congressional committees,
representatives of tobacco companies committed perjury. Even now-a-days Big
Tobacco is marketing assiduously, going so far as to use obscure parts of trade
agreements as a basis for suing governments (Australia, Britain, Uruguay and
some African countries) that pass laws to make cigarette packaging unattractive
(38-40).
Solutions
Given
that there are several causes of the present dysfunctions, no single action
could fix all the problems. Practical suggestions need to take into account the
present influence of Big Pharma, which has demonstrated that it will oppose
strenuously anything that threatens to limit drug sales, which obviously
includes tougher criteria for drug approval. Therefore initiatives for
improving matters must be based on the firmest possible evidence and should
include as public as possible a campaign for rational policies based on the
evidence. Here the Journal of Controversies in Biomedical Research can clearly
be of immediate influence. Several modifications to the process of testing and
approving drugs are so clearly proper from a technical viewpoint that
determined public pressure ought to be able to win out eventually: all results
of all clinical trials should be made publicly available in full detail; reporting
of results should include effect size, NNH, and NNT, not merely statistical
significance; and after all approvals of new drugs, there should be mandatory
systematic reporting of all possible “side” effects as well as of the achieved
efficacy at all doses. Various observers have estimated that at present no more
than 10% of adverse events come to the attention of public agencies. As
Goldacre (23), for example, has pointed out, modern electronic information
technology makes it very easy for all results of drug treatment to be archived,
whereupon algorithms can be used to raise flags if a particular drug appears to
be associated with adverse events or with lack of efficacy.
Such
actions will not be fully effective until the regulatory authorities base their
decisions strictly on the evidence. That would require a wholesale elimination
of conflicts of interest. Government agencies should not be in congenial
arrangements with drug companies (41), and civil servants engaged in research
or regulation should not be allowed to benefit from association with drug
companies (42). Such elimination of conflicts of interest will be very
difficult to achieve. At present, the FDA justifies its appointments to
advisory panels by claiming that all the qualified individuals have some sort of
research-grant or consultancy relationship with one or more drug companies.
That argument is fallacious. It requires only competent statisticians to judge
whether trial protocols are adequate and whether the statistical analysis of
results was done properly. Allowing senior officials in the National Institutes
of Health, for example, to receive payments from drug companies is clearly
harmful (42) and was also rationalized by a rather feeble excuse, that the best
people could not be attracted at salaries available in the Civil Service.
Even
under present circumstances, it ought to be possible to strengthen the criteria
by which drugs are approved in the first place. That trials need last only 6
months was put in place in the early 1990s to enable “accelerated approval” in
special cases of dire need for possibly lifesaving drugs, in the context of the
AIDS crisis. That procedure has become routine for all drug approvals, but it
ought to be possible to restrict it to the very rare situation of something like
an AIDS crisis, say for a vaccine against Ebola. Some of the current
deficiencies could be greatly ameliorated if all doctors, clinics, hospitals,
and health-insurance companies were provided with regularly updated summaries
of information gleaned from the most impartial available sources, notably the
Cochrane Collaboration which prepares meta-analyses and reviews of available
evidence. A recent initiative is David Healy’s website, RXisk (43).
After all, when law firms are able to discover quite quickly about
all-too-common and serious adverse events, it ought to be possible to provide
that information at the same time — and probably even earlier — to all
practicing physicians. The Internet and e-mail make such distribution eminently
feasible at essentially negligible cost.
Conflict of Interest
The
author declares no potential conflicts of interest with respect to research,
authorship and/or publication of this article.
References
1. Bauer HH. What's wrong with present-day
medicine? A periodically updated bibliography;
https://dl.dropboxusercontent.com/u/56983081/What%27sWrongWithMedicine.pdf, accessed 5 September 2015.
2. Claridge JA, Fabian TC. History and development
of evidence-based medicine. World J Surg 2005; 9: 547-553.
http://dx.doi.org/10.1007/s00268-005-7910-1
PMid:15827845
3. Eddy DM. The origins of evidence-based
medicine — A personal perspective. Virtual Mentor 2011; 13: 55-60.
http://dx.doi.org/10.1001/virtualmentor.2011.13.1.mhst1-1101
PMid:23134763
4. Zimerman AL. Evidence-Based Medicine: A
short history of a modern medical movement. Virtual Mentor 2013; 15: 71-76.
http://dx.doi.org/10.1001/virtualmentor.2013.15.1.mhst1-1301
PMid:23356811
5. List of Withdrawn Drugs; https://en.wikipedia.org/wiki/List_of_withdrawn_drugs, accessed 15 September 2015.
6. Bauer HH. Dogmatism in science and
medicine: how dominant theories monopolize research and stifle the search for
truth: McFarland, Jefferson (NC): 2012; 1-293 — Table 5, p. 240.
7. Anderson R. Pharmaceutical industry
gets high on fat profits. 6 November 2014;
http://www.bbc.com/news/business-28212223, accessed 7 September 2015.
8. Big Pharma has higher profit margins
than any other industry. 21 November 2014; https://www.andruswagstaff.com/blog/big-pharma-has-higher-profit-margins-than-any-other-industry, accessed 7 September 2015.
9. Corporate Watch: Pharmaceutical Industry. https://corporatewatch.org/company-profiles/pharmaceutical-industry, accessed 7 September 2015.
10. AstraZeneca. Symbicort (for US consumers.
Updated January 2015;
11. Greene JA. Prescribing by numbers:
Drugs and the definition of disease: John Hopkins University Press, Baltimore:
2007; vii-318.
12. Franklin SS, Gustin W, Wong ND, Larson
MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in
blood pressure — The Framingham Heart Study. Circulation 1997; 96: 308-315.
http://dx.doi.org/10.1161/01.CIR.96.1.308
13. Med India. Blood pressure chart; http://www.medindia.net/patients/calculators/bp_chart.asp, accessed 12 September 2015.
14. National Heart, Lung, and Blood
Institute. Description of high blood pressure. Updated 10 September 2015; http://www.nhlbi.nih.gov/health/health-topics/topics/hbp, accessed 12 September 2015.
15. Schwartz ML, Woloshin S. Changing
disease definitions: Implications for disease prevalence — Analysis of the
Third National Health and Nutrition Examination Survey, 1988–1994. Eff Clin
Pract 1999; 2(#2):76-85; Figure 3.
16. Micheel CM, Ball JR (eds). for
Institute of Medicine. Evaluation of biomarkers and surrogate endpoints in
chronic disease: National Academies Press, Washington (DC): 2010; i-31.
17. Diao D, Wright JM, Cundiff DK,
Francois Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Review,
15 August 2012, accessed 4 October 2015.
http://dx.doi.org/10.1002/14651858.CD006742.pub2
18. Martin SA, Boucher M, Wright JM, Saini
V. Mild hypertension in people at low risk. BMJ 2014; 349:g5432.
http://dx.doi.org/10.1136/bmj.g5432
19. Mack A, Balogh E, Micheel C
(rapporteurs) for Institute of Medicine. Perspectives on biomarker and
surrogate endpoint evaluation: discussion forum summary: National Academies
Press, Washington (DC): 2011; i-140.
20. Ioannidis JPA, Tzoulaki I. Minimal and
null predictive effects for the most popular blood biomarkers of cardiovascular
disease. Circ Res 2012; 110:658-662.
http://dx.doi.org/10.1161/RES.0b013e31824da8ad
21. Jarvinen TL, Sievanen H, Kannus P,
Jokihaara J, Khan KM. The true cost of pharmacological disease prevention. BMJ
2011; 342:1006-1008.
http://dx.doi.org/10.1136/bmj.d2175
22. Angell M. The truth about the drug
companies: how they deceive us and what to do about it: Random House,
http://www.penguinrandomhouse.com: 2004; 1-319.
23. Goldacre B. Bad pharma: How drug
companies mislead doctors and harm patients: Faber & Faber (Farrar, Straus
& Giroux), New York: 2013, i-426.
24. Gotzsche PC. Deadly medicines and
organised crime: How big pharma has corrupted healthcare: Radcliffe, Oxford
& New York: 2013, i-310.
25. Healy D. Pharmageddon: University of
California Press, Berkeley (CA): 2012, i-302.
26. Djulbegovic B, Hozo I, Ioannidis JPA.
Improving the drug development process: More not less randomized trials. JAMA
2014; 311:355-356.
http://dx.doi.org/10.1001/jama.2013.283742
PMid:24449311
27. Goodman SN, Redberg RF. Opening the
FDA Black Box. JAMA 2014; 311: 361-363.
http://dx.doi.org/10.1001/jama.2013.283946
PMid:24449313
28. Downing NS, Aminawung JA, Shah ND,
Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel
therapeutic agents, 2005-2012. JAMA 2014; 311:368-377.
http://dx.doi.org/10.1001/jama.2013.282034
29. Heres S, Davis J, Maino K, Jetzinger
E, Kissling W, Leucht S. Why Olanzapine beats Risperidone, Risperidone beats
Quetiapine, and Quetiapine beats Olanzapine: An exploratory analysis of
head-to-head comparison studies of second-generation antipsychotics. Am J
Psychiatry 2006; 163:185-194.
http://dx.doi.org/10.1176/appi.ajp.163.2.185
PMid:16449469
30. List of withdrawn drugs; http://en.wikipedia.org/wiki/List_of_withdrawn_drugs, accessed 15 September 2015.
31. Colquhoun D. An investigation of the
false discovery rate and the misinterpretation of p-values. R Soc Open Sci
2014; 1: 140216, accessed 12 September 2015.
http://dx.doi.org/10.1098/rsos.140216
32. Ryan, M, Combs G, Penix LP. Preventing
stroke in patients with Transient Ischemic Attacks. Am Fam Physician 1999;
60:2329-2336.
PMid:10593323
33. Carroll AE, Frakt A. How to measure a
medical treatment's potential for harm. New York Times, 2 February 2015;
http://www.nytimes.com/2015/02/03/upshot/how-to-measure-a-medical-treatments-potential-for-harm.html, accessed 9 September 2015.
34. DuBroff R, de Lorgeril M. Cholesterol
confusion and statin controversy. World J Cardiol 2015; 7:404-409.
http://dx.doi.org/10.4330/wjc.v7.i7.404
PMid:26225201 PMCid:PMC4513492
35. Wilson D. Novartis Pays $422.5 Million
in Settlement. 30 September 2010;
http://www.prescriptions.blogs.nytimes.com/2010/09/30/novartis-pays-422-5-million-in-settlement accessed 12 September 2015.
36. Grant B. Merck published fake journal.
30 April 2009;
http://www.the-scientist.com/?articles.view/articleNo/27376/title/Merck-published-fake-journal, accessed 9 September 2015.
37. Nordic Cochrane Centre, Copenhagen; http://www.cochrane.dk/about/staff.htm, accessed 9 September 2015.
38. AFTINET (Australian Fair Trade&
Investment Network Ltd). Australian High Court rules against big tobacco on
plain packaging; http://aftinet.org.au/cms/node/519, accessed 7 September 2015.
39. The Independent. Big Tobacco puts
countries on trial as concerns over TTIP deals mount. 16 September 2015;
http://www.independent.co.uk/news/business/analysis-and-features/big-tobacco-puts-countries-on-trial-as-concerns-over-ttip-deals-mount-9807478.html, accessed 16 September 2015.
40. Jolly D. Tobacco Giants sue Britain
over rules on plain packaging. 22 May 2015;
http://www.nytimes.com/2015/05/23/business/international/tobacco-plain-packaging-philip-morris-british-american-cigarettes.html?_r=0, accessed 15 September 2015.
41. Willman D. How a new policy led to
seven deadly drugs. Los Angeles Times, 20 December 2000; http://www.latimes.com/news/nationworld/nation/la-122001fda,0,4840718,full.story, accessed 16 September 2015.
42. Willman D. Stealth merger: Drug
companies and government medical research — Richard C. Eastman: A federal
researcher who defended a client's lethal drug — John I. Gallin: A clinic chief
's desire to 'learn about industry' — Ronald N. Germain: A federal lab leader
who made $1.4 million on the side — Jeffrey M. Trent: A government accolade
from a paid consultant — Jeffrey Schlom: A cancer expert who aided studies
using a drug wanted by a client. Los Angeles Times, 7 December 2003, pp. A1,
A32-5.
43. Rxisk— Making medicines safe for all of us; http://wp.rxisk.org/about, accessed 7 September 2015.